Data Collection in 3D Cell Imaging
Advances in cell culture consumables mean that spheroid and organoid culture is now highly amenable to high content screening. For example, U-bottom spheroid culture plates not only optimize growth conditions but also create uniform cell growth that sits centrally in each well.
However, to maximize the cost savings and time efficiency, scientists must be aware of the factors that influence whether or not cell imaging returns high quality data, for both multiwell plate assays and for creating a digital model from z-stack layers. As noted by In Vitro Cellular & Developmental Biology — Animal, you may not want to rely on assays developed for 2D when moving into 3D data gathering. You can't just move from the monolayer to the spheroid without optimizing for 3D.
But how?
Considering 3D in Cell Imaging
Light penetration is the number one issue to address when imaging 3D cultures, according to Rossi Bilodeau.
"The sample thickness creates an opacity barrier," she says. "You must be able to image the whole structure. Without good quality images, sampling bias can creep in."
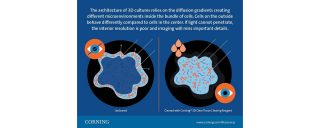
The risk of missing entire subsets of cells is a problem. The architecture of 3D cultures relies on the diffusion gradients creating different microenvironments inside the bundle of cells. Cells on the outside behave differently compared to cells in the center. If light cannot penetrate, interior resolution is poor and imaging will miss important details.
For this reason, in addition to thoroughly optimizing for cell growth characteristics and staining protocols, Rossi states it is important to prepare the 3D cultures before imaging.
The traditional route when imaging solid samples is to prepare tissue sections prior to histopathology. Although this is possible with 3D cultures, it's time-consuming and disruptive. Rossi Bilodeau suggests using a tissue clearing reagent, such as the Corning® 3D Clear Tissue Clearing Reagent, to replace this approach for 3D cultures. An advantage of the Corning 3D Clear Tissue Clearing Reagent is that it is completely compatible with high content processing; there's no need to transfer cultures and you can complete all steps in the microplate. Not only is the clearing process reversible, making 3D cultures available for further analysis, but the reagent does not alter cell morphology.
For spheroids, tissue clearing is the only step. Rossi Bilodeau notes that when dealing with organoids, there's an additional step to clear the culture matrix they're embedded in.
Quality Control
As Rossi Bilodeau describes, any imaging study requires optimization with a return to the basics for each cell type. She also advises adding in quality control steps that ensure intra- and interassay consistency.
Along with a well-optimized staining protocol with good stain penetration, predicting cell behavior helps pinpoint cell responses.
"Hypothesize cell location and thus activity within your spheroids to predict ahead where to focus on for results," she suggests. She also advises adding a nuclear stain; this will not only show penetration but also indicate cell viability and spheroid structure.
Finally, she says it's also a good idea to check in with your imaging experts as they may have specific advice on imaging modalities you can tap into for your study.